- Science Castle Research Fund
- Announcements for middle and high school students
- Information for Teachers
- Digest of Oral Presentations
- Research Tips
Toward the solution of human problems by challenging the "cell," the smallest unit of living creatures! Science Castle 2023 Kansai Digest
2024.07.22![Toward the solution of human problems by challenging the "cell," the smallest unit of living things! Digest of Science Castle 2023 Kansai Conference]</trp-post-container](https://s-castle.com/wp-content/uploads/sites/14/2024/07/b2856f6c7db2c5dd73126fd891daff64.jpg)
We will provide a digest of the "Science Castle," an academic conference for junior and senior high school students where the next generation of researchers are active, and the excitement of the field. In this issue, we report on the oral presentation by Ms. Mioko Nishi, a second-year student at Kobe High School in Hyogo Prefecture, who received the Roth Award for her presentation at the Science Castle 2023 Kansai Conference!
Affiliation and grade are as of the time of the announcement.
A dynamic world of tiny cells
My vision is "to control the ecosystems of living creatures and realize a society in which people and the Earth coexist in harmony. Once this is achieved, we will be able to solve many of the problems that humanity currently faces. To take the first step toward this goal, I believe it is necessary to understand the smallest unit. The smallest unit in living things is the cell. In fact, within that cell lies a dynamic world. Today I will show you some of it.
Did you know that there is a recycling role in cells that breaks down old matter and makes it new? I am studying autophagy, which has such a role.
Autophagy is a mechanism that degrades intracellular components on a large scale. First, a cushion-like shape called a sequestration membrane is formed, which extends and surrounds the intracellular components into a round, double-layered membrane called an autophagosome. Autophagosomes then fuse with lysosomes, cellular organelles that act like stomachs within the cell, to break down the enclosed contents.
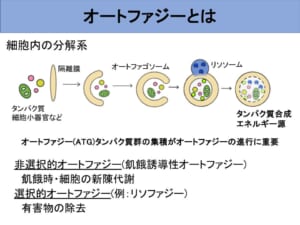
Getting rid of the bad stuff? Selective Autophagy."
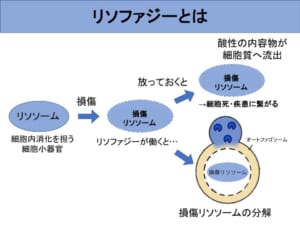
Until recently, autophagy has been thought to degrade substances in a non-selective manner. Recently, however, the existence of "selective autophagy," which selectively degrades specific substrates, has been revealed. Among autophagy, I focused on "lysophagy," which selectively removes damaged lysosomes. Lysosomes, which are cell organelles that play a role in digestion in cells, can be damaged or broken by various factors. When the lysosomal membrane breaks, acidic contents flow out into the cytoplasm, causing inflammation and oxidative stress, leading to cell death and disease. This can be compared to the human body as a whole, like a hole in the stomach that allows gastric juices to flow out. Therefore, it is important to address lysosomal damage with lysophagy and maintain intracellular integrity.
Focused on "palmitoylation" of proteins!
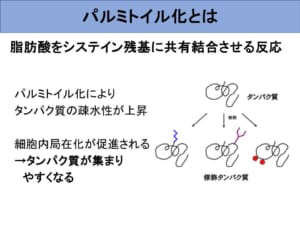
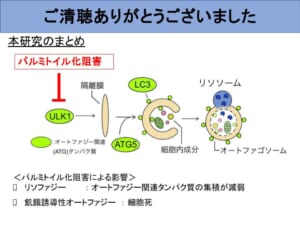
Therefore, in this study, experiments were conducted to investigate the effect of palmitoylation inhibition on autophagy. The results showed that the accumulation of proteins that function during autophagy initiation, sequestration membrane elongation, and autophagosome formation were all attenuated by palmitoylation inhibition.
Palmitoylation is a matter of cell life and death!
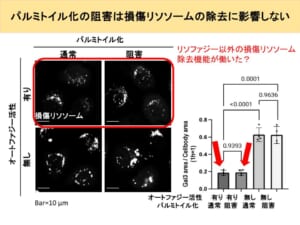
From this, it appears that lysophagy, like starvation-induced autophagy, is suppressed when palmitoylation is inhibited. In addition, when palmitoylation inhibitors were added to starved cells, the cells died. From this, it can be inferred that palmitoylation is so important for cell survival during starvation. However, from the previous experiment, we can only infer a relationship between palmitoylation and lysophagy, and we do not know how it affects the eventual repair and removal of damaged lysosomes. Therefore, we conducted an experiment to see how much damaged lysosomes remain after inducing lysosomal damage and adding a palmitoylation inhibitor. In this experiment, the damaged lysosomes were illuminated by the marker, so there is almost no fluorescence in normal cells as shown in the picture on the left. When the lysosomes are damaged, they glow brightly as shown in the middle photo.
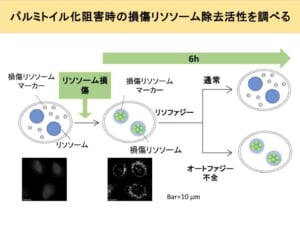
We then measure the amount of damaged lysosomes remaining in the cell 6 hours later from that point. In normal cells, damaged lysosomes are removed by lysophagy, so damaged lysosomal markers are expected to be mostly gone, while in autophagy-deficient cells, damaged lysosomal markers are expected to remain. The results show that, as expected, in cells that failed to autophagy, broken lysosomes remain in the cell compared to normal cells, thus resulting in more fluorescence.
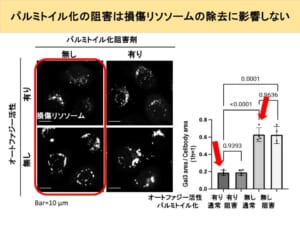
This may be because the damaged lysosomes were not properly removed by lysophagy. The graph on the right graphs the area of these fluorescences.
Second, in normal cells, there was no difference in the area of fluorescence when the palmitoylation inhibitor was added. This is contrary to expectations. It is inferred that lysophagy is not at work at the stage where proteins are illuminated and observed, because the palmitoylation inhibitor makes it difficult for the proteins to assemble.
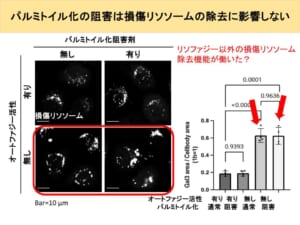
If that is the case, then adding a palmitoylation inhibitor should leave many broken lysosomes because lysophagy does not work. However, the fact that this is not the case infers that there is a mechanism other than lysophagy to repair or remove broken lysosomes.
Furthermore, there was no difference in the area of trend when palmitoylation inhibitors were added to cells that had been rendered autophagy-free.
From this, we can assume that no palmitoylation is involved in the mechanism for repairing broken lysosomes other than lysophagy, which was predicted to exist on one previous slide. From these facts, it is likely that a damaged lysosome removal pathway other than lysophagy, which does not involve palmitoylation, has been activated.
Controlling the ecosystems of living creatures toward a society in which people and the Earth coexist in harmony.
Finally, I would like to discuss future prospects. Based on this study and previous studies, we believe that inhibition of palmitoylation attenuates lysophagy and starvation-induced autophagy. Conversely, we would like to investigate what happens to autophagy when palmitoylation is promoted. I would also like to investigate how the last predicted non-lysophagic damage lysosome removal pathway, which does not involve palmitoylation, relates to the other two currently known damage lysosome response pathways. I would also like to continue my research with the aim of achieving my vision, which I mentioned at the beginning of this essay, of creating a society in which people and the Earth coexist in harmony by controlling the ecosystems of living organisms.
I would like to express my gratitude to Mr. Saeki, Prof. Yoshimori, and other members of my laboratory at Osaka University for their enthusiastic guidance in the course of this research. That concludes my presentation. Thank you very much for your kind attention.
question and answer session
Florence (Rohto Pharmaceutical Co., Ltd.):
Very interesting. I have not studied enough, but I felt again that the world of autophagy is very deep. You mentioned earlier about "people" and "living creatures." When this palmitoylation increases or decreases, what is the state of the living creature itself? For example, under what conditions does palmitoylation increase in cells? I would like to know if there is any information that autophagy is more impaired in older people and one reason for this is palmitoylation.
Nishi Mishiko:
Palmitoylation is something that occurs throughout the basic cell, so it is not in my knowledge that it is naturally reduced by disease, etc. Palmitoylation is helpful to the organism, but it also suppresses autophagy, so I feel that palmitoylation inhibition is being applied.
Florence (Rohto Pharmaceutical Co., Ltd.):
It also induces cell death, etc., so the interesting point is that it targets cancer cells, etc., isn't it? Thank you very much.
Taiyo ANZAЇ (Osaka Public University):
You are discussing the future prospects, is there anything that we can expect to find that is the cause of the failure of the removal function to work?
Nishi Mishiko:
There are two that are currently known to be predictable. One is the Escort mechanism, which removes damaged lysosomes. This is different from lysophagy in that it also removes damaged lysosomes, but does so with a little less damage or with a little more breakage. We hope to clarify in future studies that this is not an escort mechanism.
Another possibility is TFEB, a transcription factor that has been used to promote lysophagy, etc. We have recently learned that TFEB-related removal of damaged lysosomes is underway, so that may be it, but I think it is more likely that something new other than this is causing the problem. I think it is more likely that something new is the cause.
Toshihiro Ishizawa (Liverness Corporation):
This is not a question, but I would love to know what you are thinking about one or two steps beyond this presentation, such as "I would like to use this for something like this" or "I am thinking about something like this" when you are able to successfully control lysosome removal, etc., beyond the point where you have advanced your research.
Nishi Mishiko:
Yes, this research is expected to view the human lifespan, reduce aging, and such. On a personal note, I like insects. Of course, those insects also engage in autophagy, so I would like to research on extending the lifespan of insects and their relationship with parasites.
(*Honorifics omitted)